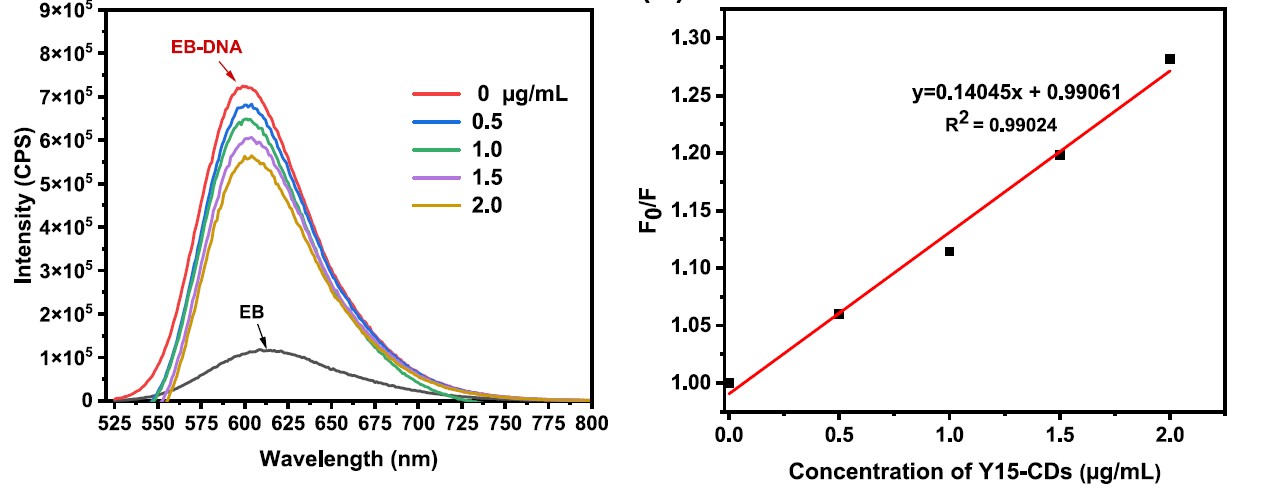
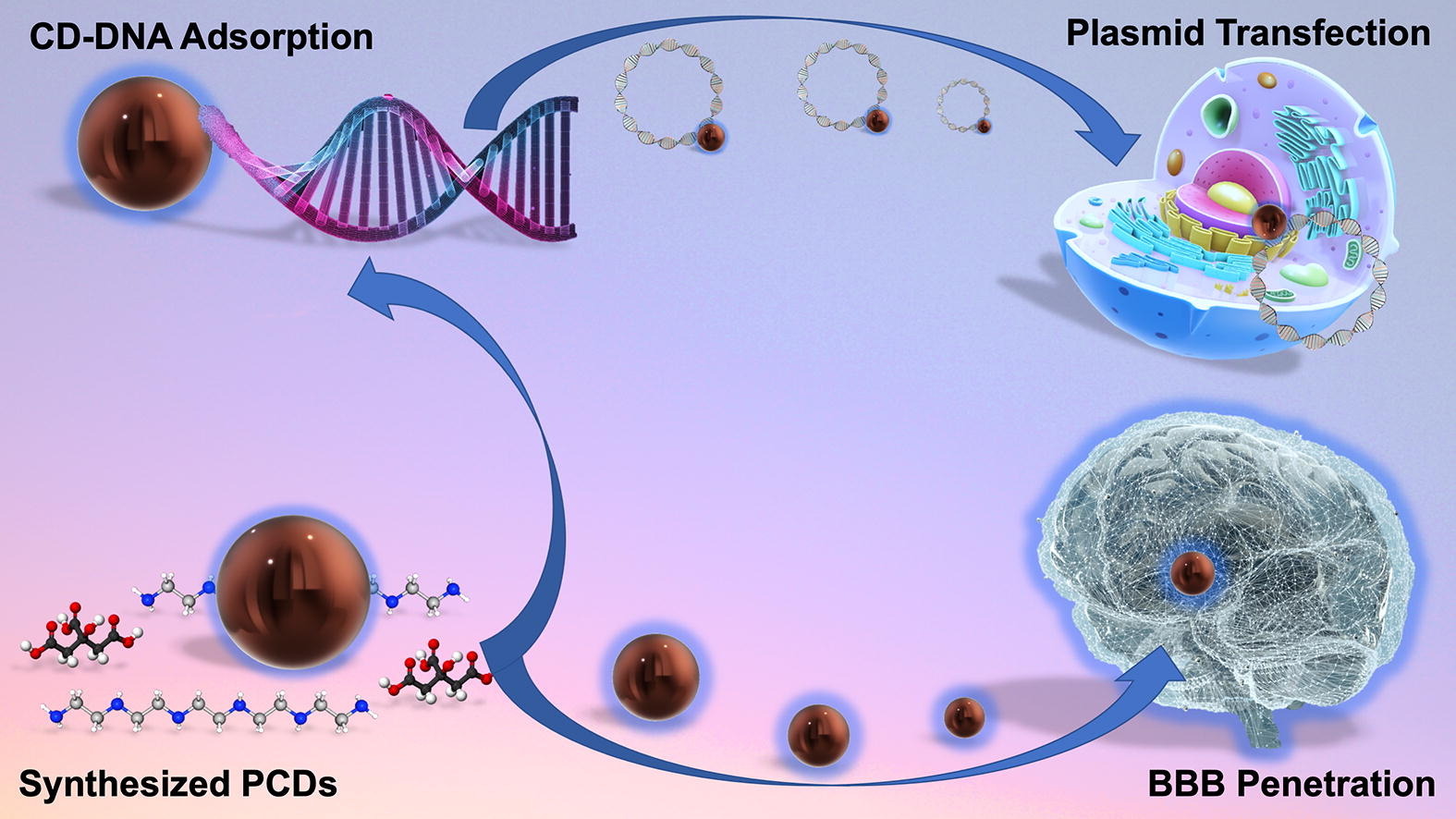
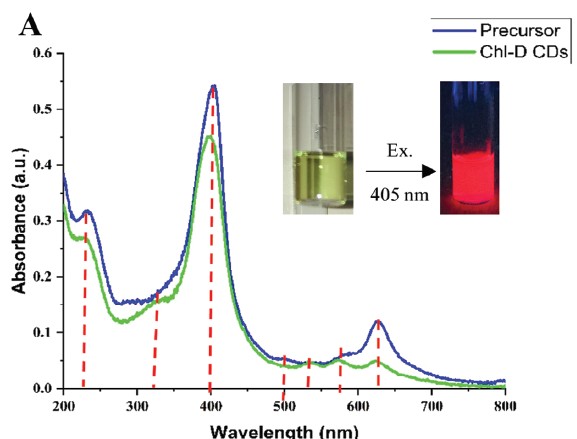
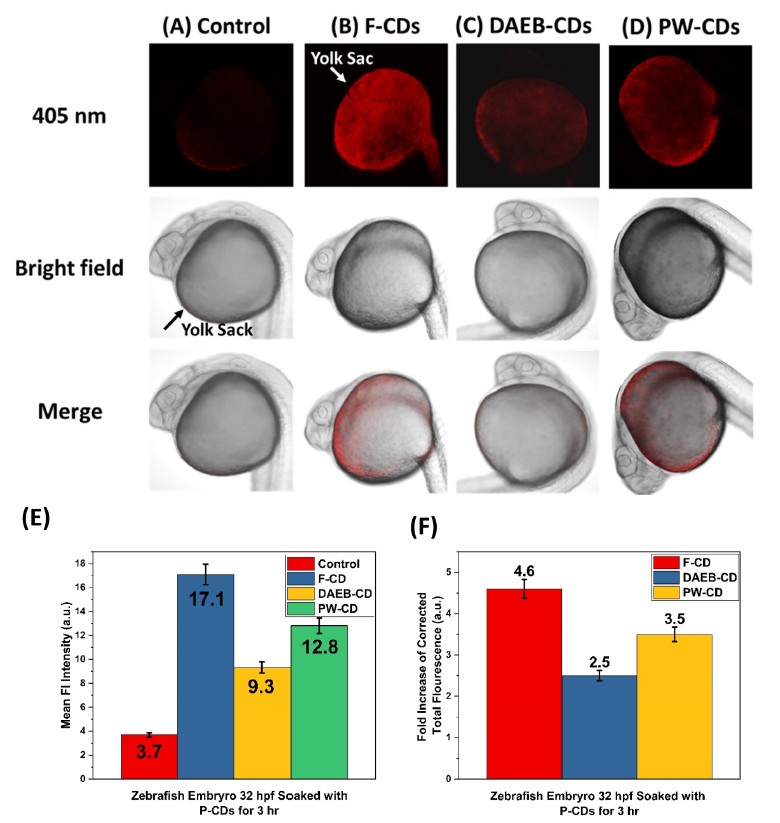
Carbon Dots
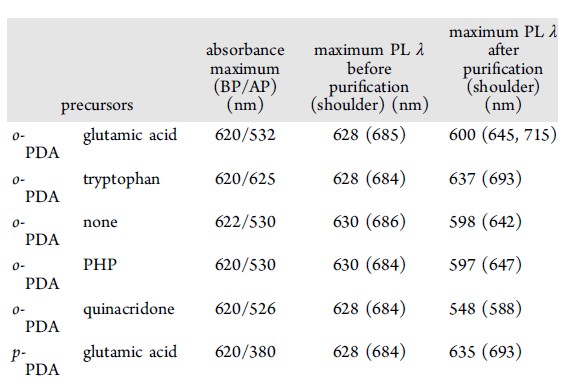
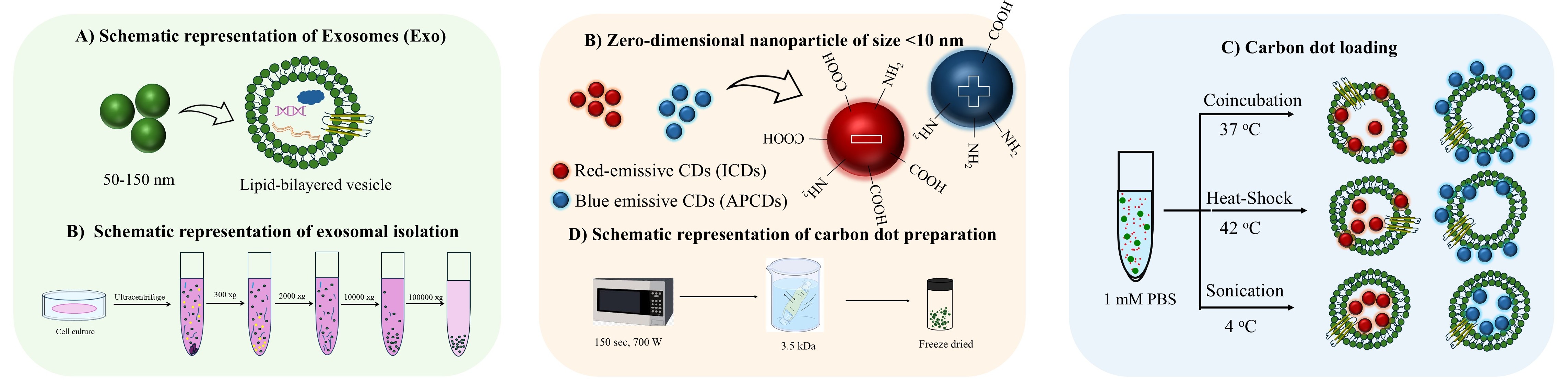
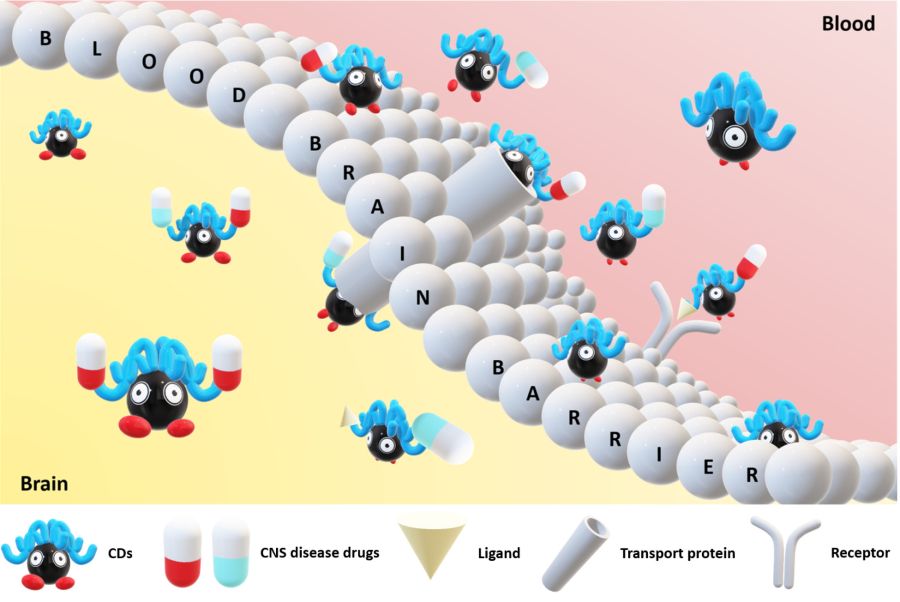
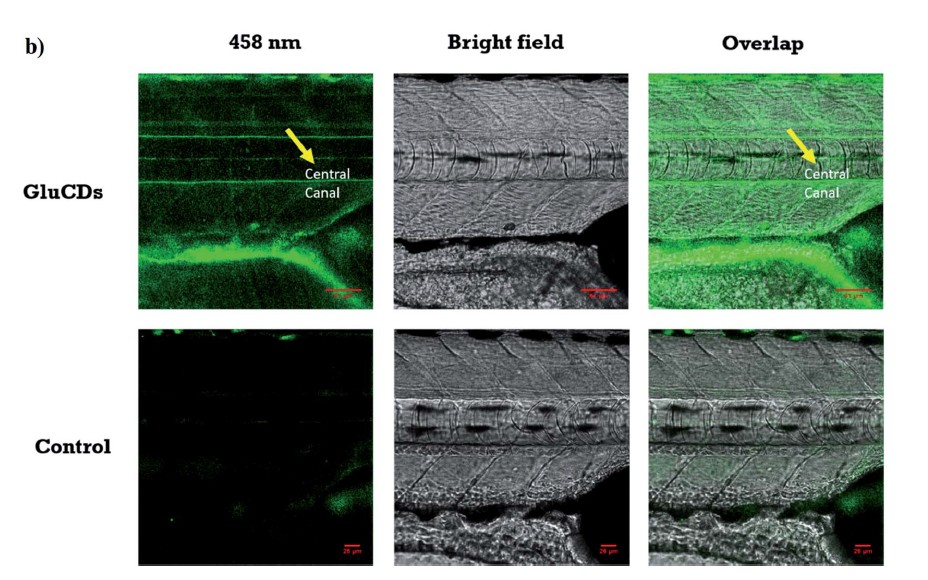
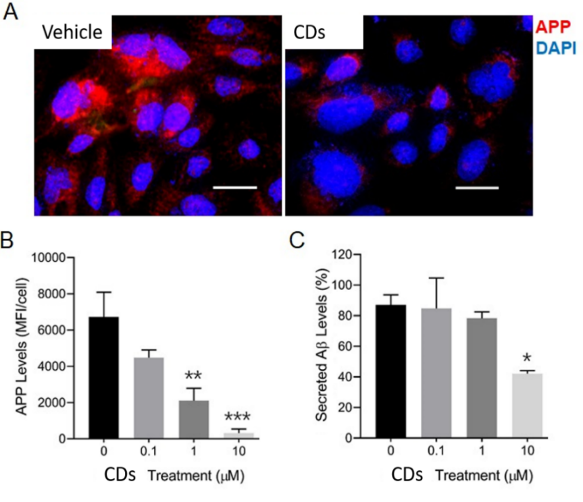
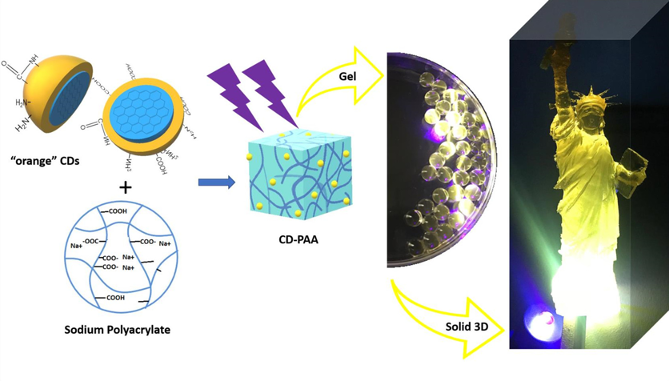
Carbon dots (CDs), first discovered in the early 21st century as biocompatible alternatives to toxic quantum dots, have rapidly emerged as versatile nanomaterials with broad biomedical and technological applications. Their potential spans cancer therapy, drug delivery across the blood–brain barrier, Alzheimer’s disease treatment, and bioimaging. However, the intrinsic short-wavelength emission of most CDs often overlaps with tissue autofluorescence, limiting their effectiveness for in vivo imaging. To overcome this, our research also focuses on engineering long-wavelength emissive CDs, particularly in the red to near-infrared (NIR) region, to enhance imaging clarity and tissue penetration. Beyond biomedical applications, our group has explored CDs in diverse fields including bone infection treatment, photocatalysis, 3D printing, thermoelectric materials, and hybrid rocket fuels. Comprehensive characterization of CDs is performed using a suite of advanced techniques such as UV–vis and fluorescence spectroscopy, FTIR, Raman, XRD, XPS, AFM, TEM, TGA, NMR, mass spectrometry, and zeta potential analysis. These efforts aim to unlock the full potential of CDs as multifunctional nanoplatforms for next-generation technologies.
(a) (b)
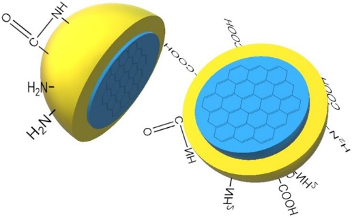
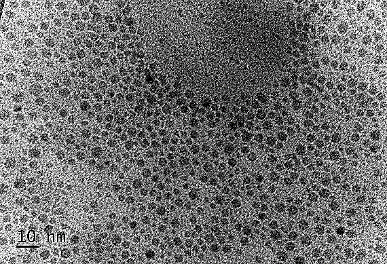
Figure 1. (a) Graphical illustration of the structure of CDs; (b) TEM image of CDs.