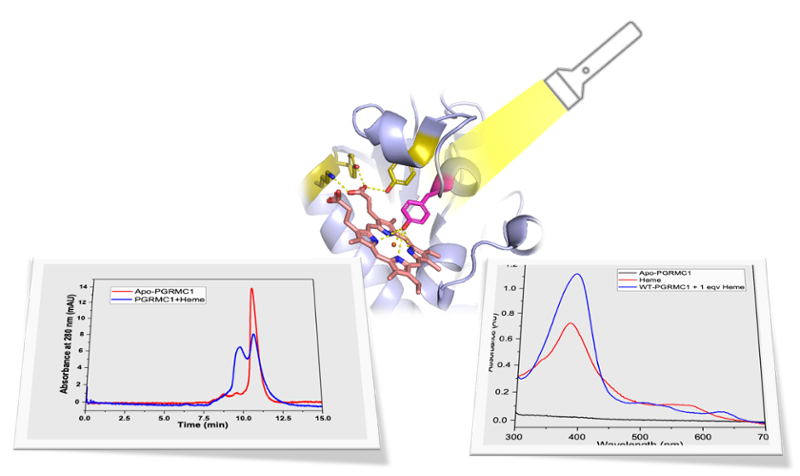
Iron is a critical component in oncogenesis due to its roles in cell cycle progression, DNA synthesis, and proliferation. However, under conditions of aberrant metal exposure, a link between high cellular iron levels and down-regulation of key tumor and metastasis suppressors has been observed. A recent report has provided the first crystallographic evidence for another contributing factor in cancer cell proliferation and metastatic processes – dimerization of the heme-containing protein progesterone-receptor membrane component 1 (PGRMC1) a membrane-associated progesterone receptor (MAPR) family. The heme-mediated dimerization of PGRMC1 is thought to be essential for the interaction of PGRMC1 with the epidermal growth factor receptor (EGFR) and drug-metabolizing cytochromes P450 to enhance tumor cell proliferation and promote chemoresistance in several cancers including endometrial, ovarian, and breast cancer. To explore these processes further, we use spectroscopic techniques as well as computational tools to do the structural characterization of the heme-binding site in PGRMC1 and gain insight into the interactions between PGRMC1 dimers and drug metabolizing P450 enzymes. As part of this effort, we are utilizing nanodiscs, which not only stabilize proteins but also provide a membrane-mimicking environment ideal for studying single-pass transmembrane proteins like PGRMC1. One of the long-term goals of this work is to combine what we learn about the PGRMC1 dimer and PGRMC1-CYP interaction surfaces with knowledge of biocompatible alternatives to devise a strategy to prevent dimerization and downstream chemoresistance.