Abstract:
Self-assembly in molecules often results with remarkable properties. Self-assembly of peptides offers a novel strategy for the synthesis of adaptable molecular architecture. Several self-assembling peptide structures have been reported to have remarkable catalytic activity, catalyzing a multitude of reactions. In this project, we use molecular dynamics simulations to explain the structural and catalytic properties of peptide assemblies with different combinations of amino acids.
Introduction:
Peptides are chains of amino acids that utilize electrostatic, hydrogen bonding, and van der Waals forces to create functional aggregations with different structures. Such aggregates have alternating hydrophobic and hydrophilic residues that are reported to have amyloid-like structures with exceptional self-healing properties with catalytic activities. In this project, I will investigate 7-peptide chain without ALF and two 9-peptide chains with ALF: a non-natural amino acid instead of Histidine, which has been reported to coordinate better than natural amino acids under near neutral conditions. I used the combination AC-IHIHIYI-NH2 for the 7-peptide chain, consisting of phenylalanine as the hydrophobic amino acid and histidine as the hydrophilic amino acid. The first 9-peptide combination, ALF-IRIRIRI-ALF, consists of isoleucine as the hydrophobic amino acid and arginine as the hydrophilic amino acid. The second combination, ALF-ITITITI-ALF, consists of isoleucine as the hydrophobic amino acid and threonine as the hydrophilic amino acid.
Computational Details:
Molecular dynamics (MD) simulations predict how every atom in a molecular system will move over time in realistic conditions. The 7-pep combination and the two 9-pep combinations had an MD done using GROMACS 2022 program. The forcefield used for the MD was AMBER99sb. MD for all combinations was done for 100 ns. Parameters for ALF was calculated using ANTECHAMBER from gaussian output. Optimization for ALF structure in gaussian used B3LYP functional with 6-31g(d) basis set.
Results & Discussion:
Alternating hydrophobic-hydrophilic sequences are reported to have amyloid like structures with exceptional self-healing properties with good catalytic activity. Introducing Arginine (R) into the mix increases the antimicrobial activity and mediates the self-healing property of the peptide chain. Arginine’s positively charged side chain will bind with isoleucine’s hydrophobic side chain to form hydrogen bonds, creating a strong interaction between the individual chains. Threonine’s polar and uncharged side chain will bind with isoleucine’s hydrophobic side chain to form hydrogen bonds as well. Metal binding residues like Histidine increases the aggregation by binding with multiple chains. Rather than using His in the 9-pep combinations, I used ALF: a non-natural amino acid which has been reported to coordinate better than natural amino acids under near neutral conditions.
Figure 1: His on the left, ALF on the right.
In the 7-pep combination AC-IHIHIYI-NH2, hydrogen bonds between the alternating PHE and HIS amino acids creates a strong interaction between the individual chains. In the 9-pep combination ALF-IRIRIRI-ALF, hydrogen bonds between the alternating ARG and ILE amino acids creates a strong interaction between the individual chains. In the 9-pep combination ALF-ITITITI-ALF, hydrogen bonds between the alternating THR and ILE amino acids creates a strong interaction between the individual chains. In each combination, hydrophobic residues prevent water from getting in, resulting in a very twisted peptide aggregate.
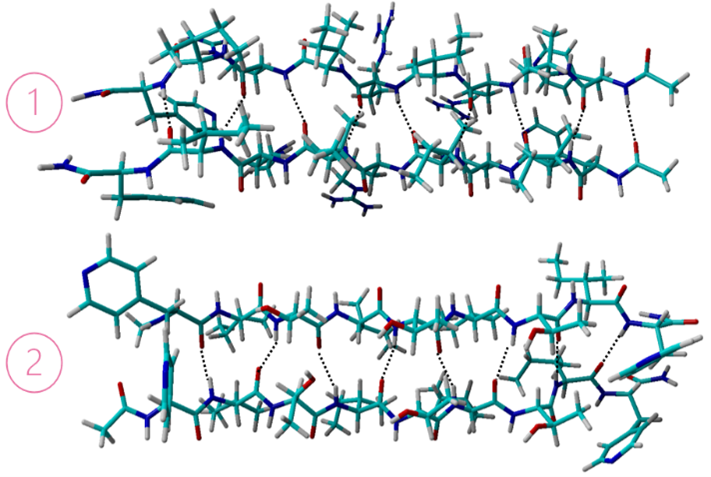
Figure 2: Hydrogen bonds are shown in ALF-IRIRIRI-ALF (1) and ALF-ITITITI-ALF (2).
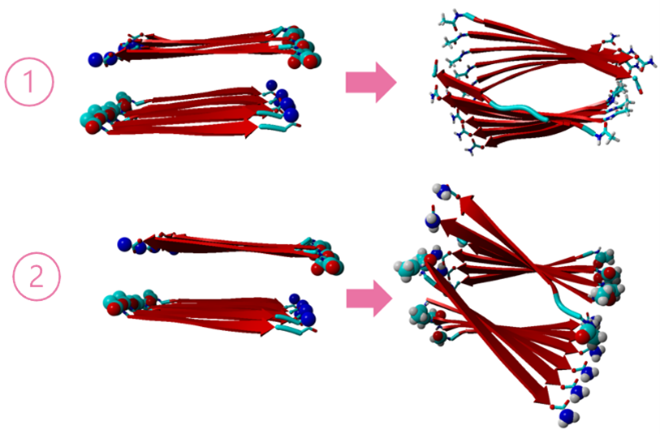
Figure 3: ALF-IRIRIRI-ALF (1) and ALF-ITITITI-ALF (2) before and after MD.
Conclusions:
Compared to ALF-IRIRIRI-ALF, ALF-ITITITI-ALF is more twisted, probably because of the smaller size of THR. The structural difference between 1 and 2 shows how important is the sequencing of the chain. The influence of sequencing is to be explored further. The equilibrated structures are without metal active sites, structures with metals are yet to be optimized. ALF binds with metal just like His. Equilibrating metal bound peptide structures is the next step of the project. Active sites with different metals and their activities are important facets of this project, and they are yet to be explored.
Acknowledgments:
I would like to acknowledge the University of Miami's Chemistry Department for giving me the chance to conduct this computational research in their lab through their collaboration with the American Chemical Society. I also want to acknowledge the American Chemical Society for its Project Seed program, which has helped me learn more about chemistry and the impact it has on nature. Finally, I want to thank Professor Rajeev Prabhakar for providing the procedures required to carry out the experiment.
References:
- D’Souza, A., Marshall, L. R., Yoon, J. H., Kulesha, A., Edirisinghe, D. I. U., Chandrasekaran, S., Rathee, P., Prabhakar, R., & Makhlynets, O. V. (2022). Peptide hydrogel with self-healing and redox-responsive properties. Nano Convergence, 9(1). org/10.1186/s40580-022-00309-7
- D’Souza, A., Yoon, J. H., Beaman, H., Gosavi, P. M., Lengyel-Zhand, Z., Sternisha, A., Centola, G., Marshall, L. R., Wehrman, M. D., Schultz, K. M., Monroe, M. B. B., & Makhlynets, O. V. (2020). Nine-Residue peptide Self-Assembles in the presence of silver to produce a Self-Healing, cytocompatible, antimicrobial hydrogel. ACS Applied Materials & Interfaces, 12(14), 17091–17099. org/10.1021/acsami.0c01154
- Marshall, L. R., Jayachandran, M., Lengyel-Zhand, Z., Rufo, C. M., Kriews, A., Kim, M. C., & Korendovych, I. V. (2020). Synergistic interactions are prevalent in catalytic amyloids. ChemBioChem, 21(18), 2611–2614. org/10.1002/cbic.202000205
- Song, R., Wu, X., Xue, B., Yang, Y., Huang, W., Zeng, G., Wang, J., Li, W., Cao, Y., Wang, W., Lu, J., & Dong, H. (2018). Principles governing catalytic activity of Self-Assembled short peptides. Journal of the American Chemical Society, 141(1), 223–231. org/10.1021/jacs.8b08893
- Zozulia, O., Dolan, M. A., & Korendovych, I. V. (2018). Catalytic peptide assemblies. Chemical Society Reviews, 47(10), 3621–3639. org/10.1039/c8cs00080h






