Abstract:
Evans Blue is a commonly used non-permeable azo dye formulation that can be used to stain to distinguish between living cells. This study introduced three-step approach to synthesizing the Evan Blue with the confirmation through high-resolution mass spectrum analysis[1]. The resulting EB-NH2 holds potential for subsequent synthesis of responsive aliphatic prodrug, which providing proving promise for the development of effective drug delivery systems toward cancer therapy.
Introduction:
Evans Blue has a high affinity for plasma albumin in the blood, so it is often used in bioscience and medical research. A long-standing goal of our project is to achieve a novel drug delivery system for highly cytotoxic PROTAC (ARV-771) by conjugating to Evans blue (EB) moiety through a tuneable responsive disulfide linker. Towards this end, we start with the synthesis of EB-NH2 for subsequent synthesis and the further biomedical application in the future.
Experimental Details[1,2]:
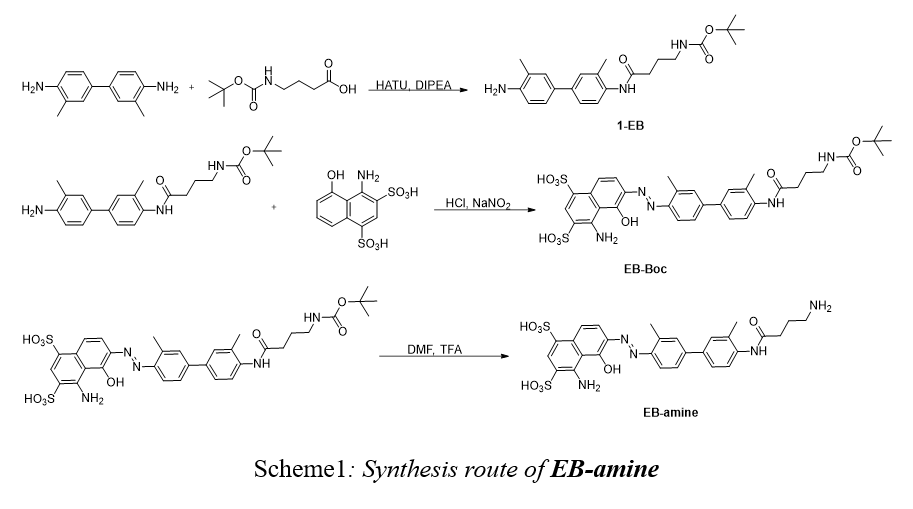
①Synthesis of compound 1-EB
o-Tolidine (2 g, 9.42 mmol) was dissolved in 10 mL DMF in a 50 mL flask. After stirring for 5 min, the 4-((tert-butoxycarbonyl)amino)butanoic acid (1 g, 4.92 mmol), HATU (3.5 g, 9.20 mmol) and N, N-diisopropylethyl-amine (DIPEA, 3 g, 23.2 mmol) was dissolved in 10 mL DMF, and then dropwise to the mixture. The reaction mixture was stirred for 6h at room temperature. Yellow mixture was washed with water (1 × 100 mL), saturated NaHCO3 aqueous solution (2 × 100 mL), and saturated brine (1 × 100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solution was concentrated by rotatory evaporator, and the residue was purified by chromatography. Gradient ethyl acetate and hexane mixture (3/1,v/v) were used as eluent. Yield: 0.9 g (48.1% yield).
②Synthesis of compound EB-Boc.
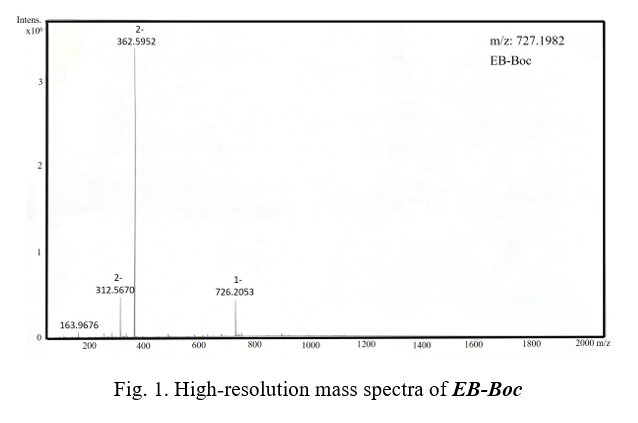
A solution of 4 mL cold 2.0 M HCl was added dropwise to a 10 mL solution of 1-EB (0.9 g, 2.26 mmol) in DMF in a 25 mL flask in an ice bath. After stirring for 15 min, 5 mL of saturated sodium nitrite was slowly added to the reaction mixture, and was stirred for another 30 min to obtain a yellow solution. The obtained solution was then added dropwise to the mixture solution of NaHCO3 (1.25 g, 14.9 mmol) and 1-amino-8-naphthol-2,4-disulfonic acid monosodium salt hydrate (1.15 g, 0.55 mmol, dissolved in 4 mL water) in an ice bath. After stirring for another 60 mins, purple reaction mixture was obtained. Purified by preparative HPLC, and lyophilized to 375 mg purple powder (yield 22.5%). HRMS (ESI) m/z: [M-H]- calculated for C33H37N5O10S2 727.1982, found 726.2053.
③Synthesis of compound EB-NH2.
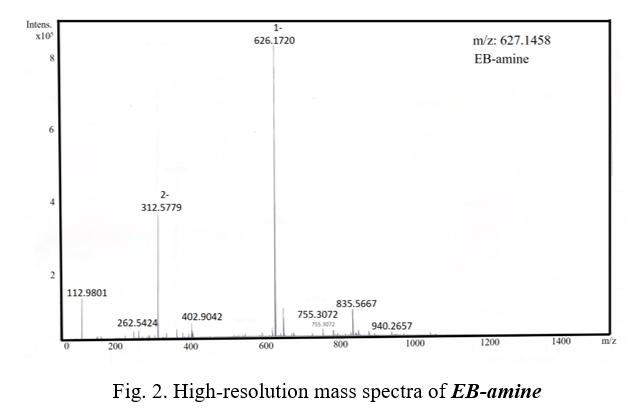
EB-Boc (375 mg, 0.516 mmol) was dissolved in 6 mL DMF in a 25 mL flask. The Trifluoroacetic acid (TFA, 3 mL, 0.039 mmol) was added. After stirring for 2 hours, the mixture was precipitated in excess of diethyl ether three times. EB-amine was obtained after vacuum drying. Yield: 107 mg (33.1% yield). HRMS (ESI) m/z: calculated for C28H29N5O8S2 627.1458, [M-H]-= found 626.0354.
Results and Discussion:
We successfully synthesized the compound EB-amine in three steps from commercially available compounds with a high purity and good yield (Scheme 1). Compound 1-EB was synthesized from o-Tolidine and 4-((tert-butoxycarbonyl)amino)butanoic acid, which was gained 0.9 gram after column chromatography. Compound EB-boc was synthesized by 1-EB and 1-amino-8-naphthol-2,4-disulfonic acid monosodium salt hydrate, it was gained 375 mg by separation by HPLC. Compound EB-amine was synthesized by deprotection of EB-boc, and then purified by being precipitated in excess of diethyl ether three times to gain 107 mg finally. The product was characterized by Mass spectrometry (Figure 1 and Figure 2).
Conclusion:
We used three steps to synthesize an albumin-binding Evan blue (EB) moiety in a high yield. The EB-amine will be further utilized for modification of PROTAC ARV-771 to achieve stimuli-responsive aliphatic molecule for drug delivery.
Acknowledgement:
This work is supported by the National Science Foundation CAREER award under grant No.2238812.
Reference:
- Shiwei Fu, Ajay Zheng, Lukun Wang, Jiuyan Chen, Bowen Zhao, Xiao Zhang, Victoria A. A. McKenzie, Zixin Yang, Roger M. Leblanc , Rajeev Prabhakar and Fuwu Zhang, Journal of Material Chemistry B, 2024,12, 6563-6569.
- Fuwu Zhang, Guizhi Zhu, Orit Jacobson, Yi Liu, Kai Chen, Guocan Yu, Qianqian Ni, Jing Fan, Zhen Yang, Frederick Xu, Xiao Fu, Zhe Wang, Ying Ma, Gang Niu, Xiaobin Zhao, and Xiaoyuan Chen, ACS Nano 201711 (9), 8838-8848.






