Abstract: This study seeks to design deep eutectic solvents (DESs) from natural hydrogen bond acceptors (HBAs) and donors (HBDs). Two DES were designed from choline, a natural, relatively ecofriendly HBD, and pyruvic acid or succinic acid, natural short chain carboxylic acid-based HBDs. Reaction between choline bicarbonate and the short chain carboxylic acid gave a DES containing cholinium ion, a HBD, a carboxylate, a HAD, and carboxylic acid, a HBD. Successful synthesis was confirmed using proton (1H) nuclear magnetic resonance (NMR). These DESs exhibit various functional properties with implications for various applications.
Introduction: Deep Eutectic Solvents are made using two or more inexpensive substances and are known to have lower melting points than the ones that make them up (Like & Panzer, 2023). Because of their lower melting points, DESs require less energy to heat, which gives them many promising applications for more sustainable technologies. However, many of them are synthesized from relatively toxic precursor, creating gap for non-toxic alternatives. This study covers the design of DESs from natural, eco-friendly biomolecules.
Experimental details: In this work, we synthesized two DESs, CAPY and CASU. The synthesis of CAPY was done by stirring Choline bicarbonate (0.01 moles) And PYruvic acid (0.04 moles) for 24 hours at room temperature. CASU was prepared by stirring Choline bicarbonate (0.01 moles) And SUccinic acid (0.02 moles) for 7 days at room temperature. Water was removed from the mixture after 24 hours using rotary evaporator set at 50 oC for 20 minutes. Then, it was left in the vacuum oven at 50 oC again for 24 hours. For both CASU and CAPY, the NMR was used to observe their structure. The solvent used for the NMR was deuterated dimethyl sulfoxide (DMSO).
Results and discussion: In CAPY, choline and pyruvic acid follow their expected peaks between 3.00 - 4.50 ppm and the DMSO peak appears at around 2.50 ppm (Figure 1). In CASU, choline bicarbonate and succinic acid follow the conventional peaks expected, but with a slight shift to the right, including the DMSO, which has a shift at 2.30 ppm instead of the expected shift at 2.50 ppm (Figure 2), which is potentially due to the contribution from water. It is evident through the decrease in strength and the broadening of the peaks in both DESs that there are strong ionic interactions and hydrogen bonding occurring. These features in the solvents typically lead to decreased lattice energy.
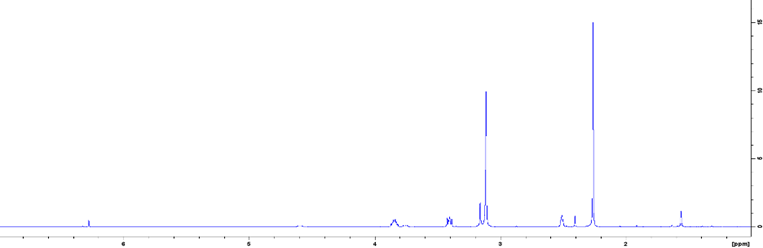
Figure 1. 1H NMR spectrum of CAPY.
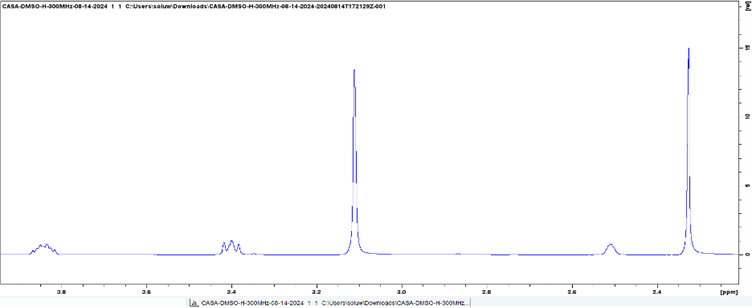
Figure 2. 1H NMR spectrum of CASU
Conclusion: It is established through the NMR that there are strong hydrogen bonding and ionic interactions present in the DES. This causes decreased lattice energy, which results in lower melting points for DES. Because of their now proven lower melting points, DES require less energy for heating. This means that they can have various applications for more sustainable technologies. An example of this is biomass processing, which requires combustion to produce fuel (US Energy Formation and Administration, 2024). Through DES, the process can be done using less energy, which is only one of the many implementations DES can have for more efficient methods.
Acknowledgements: I thank Dr. Christian Agatemor and University of Miami Young Scholars Program for giving me the opportunity to conduct research, and Gabriella Barnett, Dr. Welday Desta Weldu, and Samuel Abidemi Oluwole for guiding me on the experiment and equipment usage for the synthesis.
References:
Like, B., & Panzer, M. (2023, April 27). Defining deep eutectic solvents | School of Engineering. Tufts School of Engineering. Retrieved August 31, 2024, from https://engineering.tufts.edu/news-events/news/defining-deep-eutectic-solvents
US Energy Formation and Administration. (2024, July 30). Biomass explained - U.S. Energy Information Administration. EIA. Retrieved September 2, 2024, from https://www.eia.gov/energyexplained/biomass/





