Abstract
Medicinal plants, such as Moringa oleifera, are rising in society as a natural way to seek remedies due to their bioactive compounds and antibacterial properties. Individuals worldwide drink water unfit for human consumption with E. coli, a bacterium notorious for causing disease. This research set out to determine if moringa can inhibit the growth of E. coli by using ethanol and aqueous extracts of the moringa leaf.
Introduction
Globally, people utilize contaminated water sources as drinking water and suffer extreme health consequences or even death. E. Coli is a common bacterium found in these waters and is responsible for various water-related diseases. Rather than using chemical practices to solve this issue, natural medicinal plants can be used instead due to their phytochemical constituents. Moringa, for example, is recognized for its medicinal uses in the prevention of disease. The purpose of this study was to propose a solution to decrease the annual number of deaths from water-related diseases by determining if a moringa leaf extract can inhibit the growth of E. Coli.
Experimental details
To create the extract, moringa leaves were air-dried and macerated in 99.5% ethanol for 24 hours in an orbital shaker. The filtrate was then evaporated by use of a rotary evaporator. The extracts were created by mixing 5 mL of 99.5% ethanol with the respective amount of plant material. The following solutions of the ethanol extract were made: 5 mL of 99.5% ethanol as the control (solution A), 50 mg/mL (solution B), 200 mg/mL (solution C), and 400 mg/mL (solution D). Each solution had a different concentration by adding different amounts of the plant material to 5 mL of the ethanol. Agar plates were swabbed with E. Coli and the disks were submerged into their respective solution utilizing the Kirby-Bauer disk diffusion method. Each solution had a total of three trials by testing three disks for each solution. After the agar plate was incubated overnight, the zone of inhibition was measured for each disk in millimeters. After conducting the experiment, two additional solvents were tested, 75% ethanol and distilled water, using the same method, for comparison.
Results and discussion
The mean was calculated for each three trials of each solution. As demonstrated by Figure 1, Figure 2, and Figure 3, the average zone of inhibition for all three extracts was low and did not demonstrate a sufficient zone of inhibition to state that moringa inhibits the growth of E. Coli, based on this study. A statistical analysis was additionally done to demonstrate the significance. The data was shown to be statistically insignificant for each solution in each extract as the p-value was higher than 0.05. This may have been due to the short maceration period or the solvent used. However, based on all of the graphs, the 75% ethanol extract had the highest zone of inhibition, compared to the 99.5% ethanol extract and aqueous extract. The ethanol extracts in general had a higher zone of inhibition.
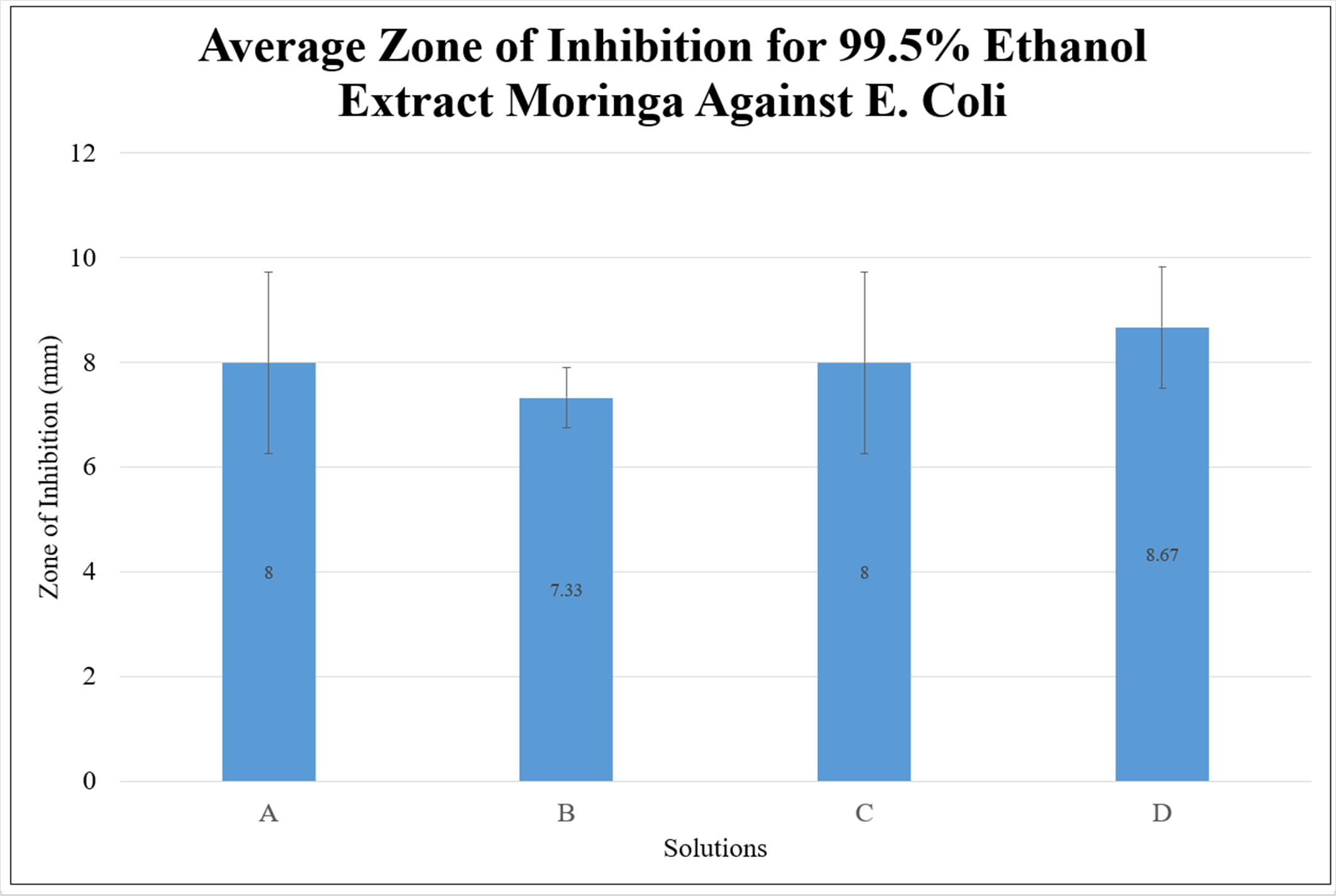
Figure 1. Graph of the Average Zone of Inhibition for 99.5% Ethanol Extract of Moringa Against E. Coli
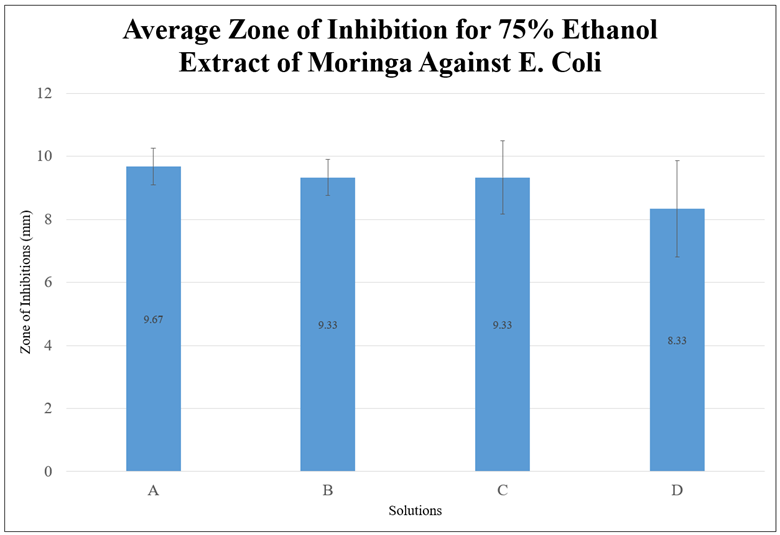
Figure 2. Graph of the Average Zone of Inhibition for 75% Ethanol Extract of Moringa Against E. Coli
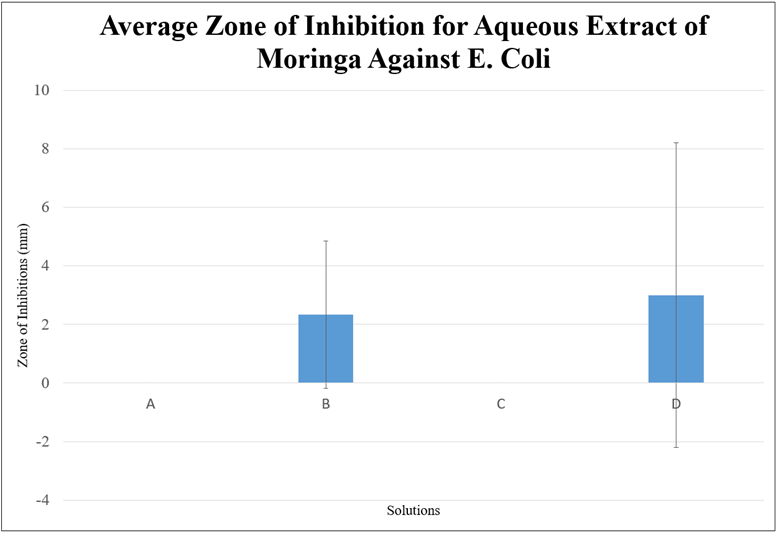
Figure 3. Average Zone of Inhibition for Aqueous Extract of Moringa Against E. Coli
Conclusion
The data from this study did not support the hypothesis that moringa extracts can inhibit the growth of E. Coli. Although the p-values showed the data to be statistically insignificant, it should be noted that the sample size was too small to have reliable data. Regardless of the outcome, this experiment poses an opportunity to conduct further research on ways to purify water and inhibit bacterial growth by using different concentrations of extract and solvents.
Acknowledgements
I would like to thank Dr. Jay for guiding me in this project and giving me access to his lab at FIU, allowing me to complete this experiment. I would also like to thank the State Science and Engineering Fair (SSEF) for recognizing my work, and Mr. Castaing for his continuous support in this project.
References
Paikra, B. K., Dhongade, H. kumar J., & Gidwani, B. (2017). Phytochemistry and Pharmacology of Moringa oleifera Lam. Journal of Pharmacopuncture, 20(3), 194–200. https://doi.org/10.3831/KPI.2017.20.022
Gopalakrishnan, L., Doriya, K., & Kumar, D. S. (2016). Moringa oleifera: A review on nutritive importance and its medicinal application. Food Science and Human Wellness, 5(2), 49–56. https://doi.org/10.1016/j.fshw.2016.04.001
Kumar, N., . P., & Pareek, S. (2021). Bioactive Compounds of Moringa (Moringa Species) (pp. 1–22). https://doi.org/10.1007/978-3-030-44578-2_28-1
Drinking-water. (2022, March 21). https://www.who.int/news-room/fact-sheets/detail/drinking-water
Walter Science School. (2018, June 5). Bacteria and E. Coli in Water | U.S. Geological Survey. https://www.usgs.gov/special-topics/water-science-school/science/bacteria-and-e-coli-water






