Abstract
Due to the slow degrading nature of plastics, our planet is littered with plastics, especially Plastic Water Bottles (PWB). PWB disposal involves recycling through harmful melting that releases hazardous gases and there is a need for a less environmentally detrimental degradation process. A study was conducted to mimic the natural weathering process by subjecting PWBs to sunlight, heating with water, and freezing. The impact of this process on Resistance to Compression (RTC) and Rebound Capacity (RC) of PWBs were studied. The study hypothesized that: (a) if PWBs are exposed to sunlight, heating with water, and freezing, then the RTC and RC would be lower than the PWBs unexposed to these factors (control); and (b) if PWBs were frozen and immediately heated with water (with or without sunlight), then the RTC and RC would decrease more than the non-heated PWBs. The study had eight treatments where PWBs were treated/untreated with sun, water, freezing, and heating and the study had three replications. Key water quality attributes (pH, Electrical Conductivity (EC), and Total Dissolved Salts (TDS)), RTC, and RC were measured. Results showed that there was no difference in pH among water samples. However, EC was 200% higher in heat treated water samples compared to the non-heated samples but all treatments had higher EC compared to the initial water samples (control). TDS was 100-150% higher in heated water samples compared to the non-heated samples but all treatments had higher TDS compared to the control. When all PWBs were subjected to 68.95 kPa pressure, PWBs exposed to sunlight and water were compressed to 3mm thickness compared to 8mm observed in control-PWBs. PWBs not exposed to sunlight but exposed to water and freezing were compressed to 7mm. PWBs exposed to sunlight and water lost 42-45% of their RC compared to unexposed PWBs and control. PWBs not exposed to sunlight but exposed to water and freezing had the same RC as that of control. PWBs exposed to sunlight, freezing, and heating with water had 34% lower RC compared to PWBs exposed only to sunlight. The results showed that PWBs lose their resistance and become fragile 50% faster whenever they are exposed to sunlight, heating with water, and freezing in a controlled environment. These results showed that the first hypothesis was correct because sunlight, water, heating, and freezing played an important role in rapid PWB degradation. The second hypothesis was not correct because Florida’s high ambient temperature had the same effect of heated treatments when exposed to sunlight. This is a low-cost and environmentally sound approach that can be used by local governments or individual owners to safely dispose plastics.
Introduction
Pyrolysis is a common recycling process which requires recycled plastics to undergo high-temperature melting to create new plastic objects. This process releases harmful gaseous substances that contribute to air pollution. Without pyrolysis, plastics take hundreds of years to degrade naturally, a process dependent on their thickness and the type of chemicals used to manufacture them. There are various types of plastics, and the most common is single-use water bottles. Water bottles are made of polyethylene terephthalate (PET). Scientists postulate that materials made of PET would take more than 400 years to decompose naturally. Thus, plastics staying environment will lead to widespread contamination of water bodies if they are thrown in ocean, lakes, river, etc. Water bottles discarded on land cause similar damage by remaining intact for decades, affecting the quality of soil and ground water. Therefore, there is a need to degrade plastic bottles quickly to reduce damage to our environmental resources.
Scientists are experimenting with various methods of plastic degradation, such as larvae and marine bacteria. The key issue is the unknown effects of solving plastic degradation with these unreliable methods. In other words, given the possibility of very slow degradation, scientists are still debating what would happen if these bacteria and larvae do not feed on plastics, and start feeding on crops instead. There are enough data to show that water bottles thrown in water bodies lose their buoyancy, submerge, and become brittle. This process ultimately leads to disintegration of plastic bottles into micro- or nano-particles. These plastic particles are ingested by fish and other marine animals, and ultimately could end up consumed by humans, causing deleterious health effects.
The question that remains to be answered is, if water bottles can become brittle under water, what are the factors that could enhance the chemical process that results in expedited degradation? Identifying these factors will guide us to develop methods to degrade plastics faster in a controlled environment. Scientists theorized that sunlight would lead to plastic degradation through ultraviolet rays, but there is a lack of understanding whether the sunlight is a catalyst or an actual factor in plastic degradation. If the sunlight is a catalyst, would placing plastic bottles in water enhance degradation? Due to the lengthy degradation process, researchers have yet to link plastic brittleness to in the presence of sunlight and water. Exposing plastics to water in the presence of sunlight might enhance degradation. In addition, prolonged sunlight exposure on water temperature could decrease the structural integrity of plastics. This weathering process might impact plastic degradation through expansion and contraction. However, there is lack of knowledge and research conducted to explain the impact of water on plastics in the presence of sunlight. There is also a need to understand water’s reaction on plastics through changes in chemical properties of water. These factors might influence the resistance to compression (RTC). RTC is an important physical property of plastics that is inversely related to brittleness or degradation potential. There is a need to degrade these plastics faster whenever recycling is not possible. Plastic water bottles can be subjected to various chemical reactions. Plastics exposed to natural weather conditions such as sun, rain, water, heat, cold temperature, etc., may degrade faster, but scientists are yet to study the impact of natural factors on plastics.
Problem Statement
- Can we artificially induce a weathering process to accelerate degradation of plastic water bottles by reducing their resistance to compression?
- Does exposing plastic water bottles to sun, water, heat, and freezing have any impact on plastic water bottles’ ability to rebound through chemical reactions?
Hypotheses
- If plastic bottles were exposed to sunlight, water, heat, and freezing, then the plastic’s Resistance to Compression (RTC) and Rebound Capacity (RC) would be lower than the unexposed water bottles
- If plastic bottles were frozen and heated immediately with or without sunlight, then the plastic water bottles would lose RTC and RC faster than non-heated water bottles
Materials
- 72 Plastic water bottles (Kirkland® Brand)
- 11 Aluminum trays (51.88 cm x 32.5 cm x 6.25 cm)
- One 18.75-liter bucket
- Four 60 cm x 90 cm clear glass sheet
- 21 Glass Bottles
- Baoshishan Push/Pull Force Tester for Compression
- 324 Sterno 2.25-Hour Methanol Gel Chafing Fuel
- Two wooden planks (7.5 cm x 45 cm)
- Two rulers 15 cm x 3 cm
- One pH meter
- One Electrical Conductivity meter
- One HM® digital Total Dissolved Solid Meter
- One DeWalt®XL Trigger Clamp
- One box cutter
- One refrigerator with freezer
- One tube of permanent glue made by 3M®
Experimental Details
- Treatments: Eight
- Treatment#1: Six bottles kept in the house without exposure to sun, water, or heat
- Treatment#2: Six bottles placed in a tray without water but exposed to sun
- Treatment#3: Six bottles placed in a tray filled with city water and exposed to sun
- Treatment#4: Six bottles placed in a tray filled with city water, exposed to sun, and heated once a week
- Treatment#5: Six bottles placed in a tray filled with city water, exposed to sun, and heated twice a week
- Treatment#6: Six bottles placed in a tray filled with city water, not exposed to sun but heated once a week
- Treatment#7: Six bottles placed in a tray filled with city water, not exposed to sun but heated twice a week
- Treatment#8 Six bottles placed in a tray filled with city water but not exposed to sun or heat
Control: Treatment#1 for RTC and RC and Initial water quality for pH, Electrical Conductivity (EC), and Total Dissolved Solids (TDS).
Constants: same bottle, same trays, same water
Independent Variables: Heating Frequency and Exposure to Sun, Water, and Freezing
Dependent Variables: Water pH, EC, TDS, RTC and RC
Replications: Three
Study Period:
- May 25, 2020 to September 26, 2020
Procedures
- Place bottles in trays and fill the trays with city water depending on treatments
- Test water for pH, Electrical Conductivity (EC), and Total Dissolved Solids (TDS) before the experiment
- Cover all outside replications with glass sheets, with space at the edges, to drain rain water
- Heat Treatment #4 and #6 on Saturdays and Treatment #5 and #7 on Saturdays and Sundays using Methanol gel for two hours from 8 A.M. to 10 A.M. Repeat heating every week.
- Freeze all bottles from Treatment#3 to Treatment#8 for 24 hours on Fridays
- Refill all trays with water every week on Tuesdays.
- Leave trays in the same location until September 27, 2020
- Collect water samples from each tray and dry all water bottles
- Measure pH, EC (µS/cm), and TDS (ppm) of water samples at the end of the experiment
- Build two wooden planks to cover the widths of bottles when compressed
- Cut the spout of all water bottles
- Permanently glue the rulers on the upper and lower lips of the clamps
- Place one wooden plank on the bottom lip of the DeWalt Trigger Clamp, place the second wooden plank on top, and exert pressure by squeezing the trigger. (The maximum compression that can be applied to keep the wooden planks air-tight is 68.95 kPa)
- Place each water bottle on the bottom wooden plank and place the second wooden plank on top of the bottle.
- Pull the clamp trigger to exert 68.95 kPa on the wooden planks to compress the bottles
- Measure the gap between wooden planks
- Loosen up the clamp to release the bottle and leave the bottle aside for 15 minutes to rebound
- Place the rebounded bottle on the bottom wooden plank, place the second wooden plank on top of the bottle, and gently slide the upper lip of the clamp to rest on the upper plank to hold the bottle without exerting any pressure
- Measure the gap between wooden planks
- Conduct statistical analysis using the method suggested by the South Florida Regional Science and Engineering Fair.

Results
Water samples from all treatments were analyzed at the end of the experiment for pH, Electrical Conductivity (EC), and Total Dissolved Solids (TDS). The initial pH value of water sample was 7.93 before the experiment. The lowest pH recorded after the experiment was 7.78 observed in treatments unexposed to sunlight and heat and the highest pH recorded was 8.16 observed in treatments exposed to sunlight but heated once a week.
The EC of the initial sample before the experiment was 343.33 µS/cm. Among the treatments exposed to sunlight, the lowest EC was 704.67 µS/cm observed in treatments without any heating but the heated treatments had 1,396.67 and 1,550 µS/cm respectively for once- and twice-a-week heating. Among the treatments unexposed to sunlight, the lowest EC was 663 µS/cm observed in treatments in unheated treatments but the heated treatments had 1,200 and 1,753.33 µS/cm for once- and twice-a-week heating respectively. There were no differences in EC due to sunlight but the differences were noticeable between heated and non-heated treatments. Treatments heated twice-a-week had higher EC compared to once-a-week heating.
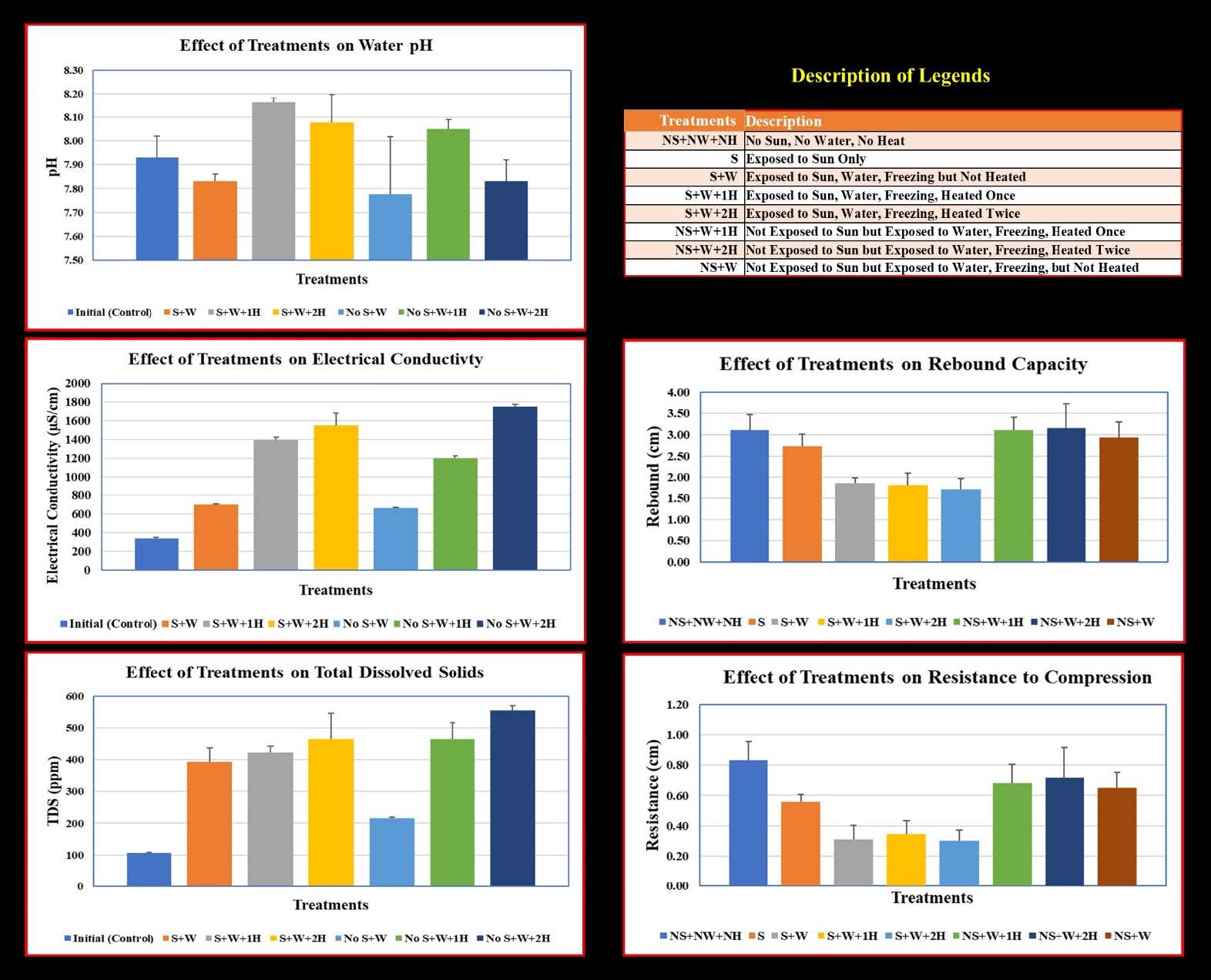
The TDS of the initial sample before the experiment was 106.67 ppm. Among the treatments exposed to sunlight, the lowest TDS was 373 ppm observed in treatments without any heating but the heated treatments had 421.67 and 465.33 ppm for once- and twice-a-week heating respectively. Among the treatments unexposed to sunlight, the lowest TDS was 215.67 ppm observed in the unheated treatment but the heated treatments had 431 ppm and 554.67 ppm for once- and twice-a-week heating respectively. There were no differences in TDS due to sunlight but the differences were noticeable between heated and non-heated treatments. Treatments heated twice-a-week had higher TDS compared to once-a-week heating.
Among PWBs compressed with equal amount of pressure, highest Resistance to Compression (RTC) of 0.83 cm was observed in control treatment where bottles were unexposed to sunlight, water, freezing, and heat but the lowest RTC of 0.30 cm was observed in PWBs exposed to sunlight, water, freezing, and twice-a-week heating. Among PWBs exposed to water, freezing, and heating, RTC ranged from 0.30 to 0.34 cm in PWBs exposed to sunlight whereas the PWBs unexposed to sunlight ranged from 0.65 to 0.72 cm. PWBs exposed to sunlight had 59 to 64% lower RTC compared to the control but for the PWBs unexposed to sunlight the reduction was only 14-22% lower than the control.
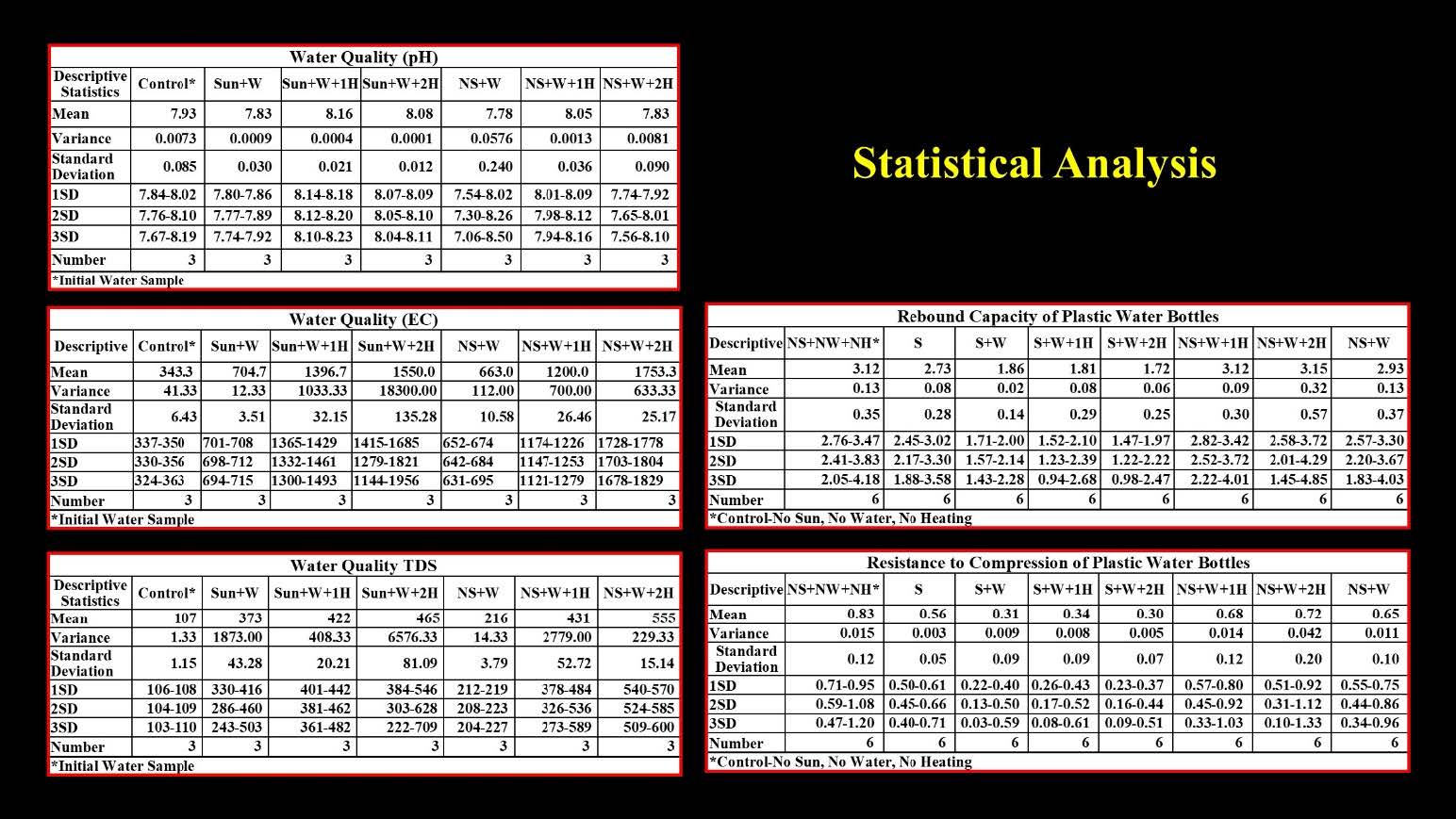
Ability of PWBs to rebound (Rebound Capacity, RC) after compression showed that the PWBs in the control treatments rebounded to 3.12 cm but the lowest amount of rebound was 1.72 cm observed in PWBs exposed to sunlight, water, and twice-a-week heating. Among PWBs exposed to water and heating, rebound capacity ranged from 1.72 to 1.86 cm in PWBs exposed to sunlight whereas the PWBs unexposed to sunlight ranged from 2.93 to 3.15 cm. PWBs exposed to sunlight had 41-45% lower RC compared to the control but for the PWBs unexposed to sunlight the reduction was only 6% lower than the control.
Discussion
The experiment showed that chemical properties of water did not impact the RTC and RC of PWBs. Although water pH did not change among treatments exposed PWBs to water, EC and TDS increased in those treatments proportionate to the heating frequencies but higher EC and TDS did not impact RTC and RC of these treatments. Data showed that PWB exposure to sunlight, water, heating, and freezing resulted in lower RTC and RC in these PWBs compared to the control and other treatments unexposed to sunlight. Consistent reduction in RTC and RC can occur in PWBs whenever the combination of sunlight, water, and freezing are used to degrade PWBs. Lack of differences in RTC and RC among PWBs treated with once- or twice-a-week heating and without heating proved that heating or its frequency did not have any positive impact in Florida where abundant sunlight causes natural heating.
Exposure to heating positively impacted RTC and RC but only in the presence of sunlight. There was 50% reduction of RTC and RC when PWBs were exposed to sunlight, water, and freezing. Although heating did not show any difference in RTCs and RCs, Florida’s high ambient temperature might have increased water temperature which is similar to heating every day. Lack of differences in RTCs and RCs of PWBs exposed to sunlight and water with no heating or once- and twice-a-week heating is a proof that impact of heating frequencies on RTCs and RCs is compounded by the natural hot weather in Florida. However, the reduction in RTCs and RCs of PWBs kept outside was about 50% lower than comparable treatments. Therefore, based on the data shown above, it can be concluded that exposing of PWBs to sunlight, water, and freezing in South Florida can degrade plastics 50% sooner than untreated PWBs. These results showed that the hypotheses were correct because sunlight, water, heating, and freezing played an important role in rapid PWB degradation. Artificial heating could play an important role in PWB degradation in areas outside Florida.
Application
Cities and local governments in Florida can rapidly degrade PWBs in a contained area by building a degradation plant where PWBs can be exposed to sunlight, water, and freezing. Expedited degradation can be used to convert PWBs into plastic particles. These plastic particles can be used in the nursery industry to aerate the root systems of potted plants. Containerized plants require sufficient aeration in soil mixture so that water can drain quickly without causing any root damage. Soil mixtures with plastic particles will provide the perfect growing conditions for potted plants.
References
Barry, Carolyn. “Plastic Breaks Down in Ocean, After All-And Fast.” National Geographic, 20 Aug. 2009, www.nationalgeographic.com/news/2009/8/plastic-breaks-down-in-ocean-after-all-and-fast/.
Compression and Tension Strength of Some Common Materials, www.engineeringtoolbox.com/compression-tension-strength-d_1352.html.
Compressive Strength Testing of Plastics, www.matweb.com/reference/compressivestrength.aspx.
Creighton, Jolene. “Infographic: Here's How Long Your Trash Will Be Around.” Futurism, Futurism, 8 Feb. 2014, futurism.com/plastic-decomposition.
Drahl, Carmen. “Plastics Recycling with Microbes and Worms Is Further Away than People Think.” Chemical & Engineering News, American Chemical Society, 18 June 2018, cen.acs.org/environment/sustainability/Plastics-recycling-microbes-worms-further/96/i25.
Gewert, Berit, et al. “Pathways for Degradation of Plastic Polymers Floating in the Marine Environment.” Environmental Science. Processes & Impacts, U.S. National Library of Medicine, Sept. 2015, www.ncbi.nlm.nih.gov/pubmed/26216708.
“Home.” Cole, 10 Apr. 2019, www.coleparmer.com/tech-article/uv-properties-of-plastics.
Miandad, et al. “Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries.” Frontiers, Frontiers, 22 Feb. 2019, www.frontiersin.org/articles/10.3389/fenrg.2019.00027/full.
Plastics Standards, www.astm.org/Standards/plastics-standards.html.
Scalenghe, Riccardo. “Resource or Waste? A Perspective of Plastics Degradation in Soil with a Focus on End-of-Life Options.” Heliyon, Elsevier, 10 Dec. 2018, www.sciencedirect.com/science/article/pii/S2405844018301336.
Shah, Aamer Ali, et al. “Biological Degradation of Plastics: A Comprehensive Review.” Biotechnology Advances, Elsevier, 26 Jan. 2008, www.sciencedirect.com/science/article/abs/pii/S0734975008000141.
Solutions, Pollution. “What Is Plastic Photodegradation?” Pollution Solutions Online, www.pollutionsolutions-online.com/news/waste-management/21/breaking-news/what-is-plastic-photodegradation/35801.
Staff, Creative Mechanisms. “The Eleven Most Important Types of Plastic.” The Eleven Most Important Types of Plastic, www.creativemechanisms.com/blog/eleven-most-important-plastics.
“This Caterpillar Can Digest Plastic.” Nature News, Nature Publishing Group, 24 Apr. 2017, www.nature.com/articles/d41586-017-00593-y.
Tosin, Maurizio, et al. “Laboratory Test Methods to Determine the Degradation of Plastics in Marine Environmental Conditions.” Frontiers in Microbiology, Frontiers Research Foundation, 21 June 2012, www.ncbi.nlm.nih.gov/pmc/articles/PMC3380294/.
“Toxicological Threats of Plastic.” EPA, Environmental Protection Agency, 19 June 2017, www.epa.gov/trash-free-waters/toxicological-threats-plastic.
Urbanek, Aneta K, et al. “Degradation of Plastics and Plastic-Degrading Bacteria in Cold Marine Habitats.” Applied Microbiology and Biotechnology, Springer Berlin Heidelberg, Sept. 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC6132502/.
Wang, Luyi, et al. “A Strong and Tough Interpenetrating Network Hydrogel with Ultrahigh Compression Resistance.” Soft Matter, The Royal Society of Chemistry, 14 Apr. 2014, pubs.rsc.org/en/content/articlelanding/2014/sm/c4sm00206g#!






