Abstract. The investigation sought to determine if heavy metal accumulation, chromium sulfate or nickel sulfate, would cause a decrease in magnesium content within Brassica juncea, Brassica oleracea aceophala and Brassica oleracea gongylodes. The effect of heavy metals Nickel Sulfate and Chromium Sulfate on the magnesium content on plants of the genus Brassica was tested to determine possibilities of a prospective phytoremediator. Chromium Sulfate and Nickel Sulfate were introduced daily to the Brassica plants for thirteen days. The magnesium content of each plant was measured, and data did support the hypothesis that plants exposed to heavy metal accumulation will have a decrease in magnesium content.
Introduction. Heavy metal contamination is a very serious environmental issue inhibiting plant productivity and affecting human health. Soils contain a plethora of heavy metals and trace metals. Hyperaccumulation is one of the fundamental characteristics for plants usually used for phytoremediation, which is the ability to remove or render toxic environmental contaminants harmless. A plant must handle high levels of the element in its roots and shoot cells. There must be a fast intake rate of the element. The effect of heavy metals Nickel Sulfate and Chromium Sulfate on the magnesium content on plants of the genus Brassica was tested to see if this genus was a prospective phytoremediator. After the test groups, Nickel Sulfate and Chromium Sulfate were introduced to the plants, the magnesium content was tested for parts per million. Magnesium is an essential ion in process of photosynthesis.
Procedures. Sixty four plants within the genus Brassica were tested under LED lights setting. Each species of plant was exposed to either Nickel Sulfate or Chromium Sulfate for thirteen days. One group, the control was not exposed to any metals. After the thirteen day period, each plant was tested for the magnesium content within a certain mass content.
|
Table 1.4 Descriptive Information
Red Russian Kale
Magnesium Content (ppm)
|
Controlled
|
Nickel Sulfate
|
Chromium Sulfate
|
|
Mean
Variance
Standard Deviation
1 SD (68% Band)
2 SD (95% Band)
3 SD (99% Band)
Number
|
1085
10500
102.47
982.53 – 1187.47
880.06 – 1289.94
777.59 – 1392.41
6
|
700
42000
204.94
495.16 – 904.94
290.12 – 1109.88
85.18 – 1314.82
6
|
600
18000
134.16
465.84 – 734.16
331.68 – 868.32
197.52 – 1002.48
6
|
|
Results of F-Test
|
F = 16.74
Critical F=3.6823 < 16.74
|
Df =2,15
rejected
|
p>0.10
α = 0.05
|
|
Table 1.5 Descriptive Information
Kohlrabi
Magnesium Content (ppm)
|
Controlled
|
Nickel Sulfate
|
Chromium Sulfate
|
|
Mean
Variance
Standard Deviation
1 SD (68% Band)
2 SD (95% Band)
3 SD (99% Band)
Number
|
987.5
18937
137.61
849.89 – 1125.11
712.28 – 1262.72
574.67 – 1400.33
6
|
432.5
8857.5
94.11
338.39 – 526.61
244.28 – 620.72
150.17 – 714.83
6
|
700
15000
122.47
577.53 – 822.47
455.06 – 944.94
332.59 – 1067.41
6
|
|
Results of F-Test
|
F = 32.40
Critical F=3.6823 < 32.40
|
Df =2,15
rejected
|
p>0.10
α = 0.05
|
|
Table 1.6 Descriptive Information
Mustard, India
Magnesium Content (ppm)
|
Controlled
|
Nickel Sulfate
|
Chromium Sulfate
|
|
Mean
Variance
Standard Deviation
1 SD (68% Band)
2 SD (95% Band)
3 SD (99% Band)
Number
|
1100
6000
77.46
1022.54 – 1177.46
945.08 – 1254.92
867.62 – 1332.38
6
|
700
6000
77.46
622.54 – 777.46
545.08 – 854.92
467.62 – 932.38
6
|
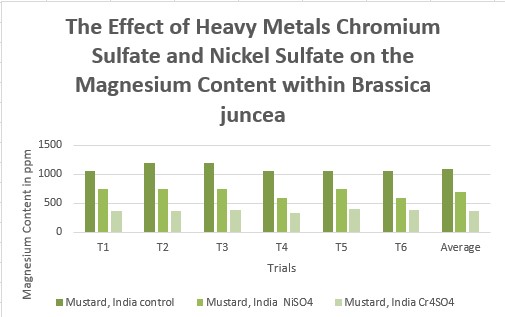
375
720
26.83
348.17 – 401.83
321.31 – 428.66
294.51 – 455.49
6
|
|
Results of F-Test
|
F = 186.62
Critical F=3.6823 < 186.62
|
Df =2,15
rejected
|
p>0.10
α = 0.05
|
|
Table 1.3 Descriptive Information
Chromium Sulfate Group
Magnesium Content (ppm)
|
Red Russian Kale(Brassica oleracea acephala
|
Kohlrabi (Brassica oleracea gongylodes)
|
Mustard, India (Brassica juncea)
|
|
Mean
Variance
Standard Deviation
1 SD (68% Band)
2 SD (95% Band)
3 SD (99% Band)
Number
|
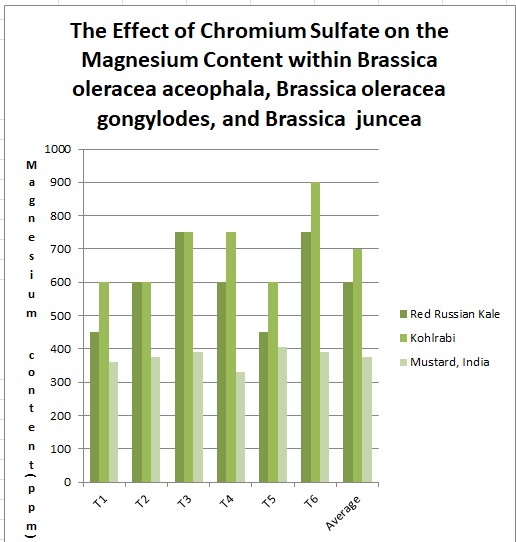
600
1800
42.43
557.57 – 642.43
515.14 – 684.86
472.71 – 727.29
6
|
700
111,000
333.17
366.83 – 1033.17
33.66 – 1366.34
-299.51 – 1699.51
6
|
375
720
26.83
348.17 – 401.83
321.34 – 428.66
294.51 – 455.49
6
|
|
Results of F-Test
|
F = 3.84
Critical F=3.6823 < 3.84
|
Df =2,15
rejected
|
p>0.10
α = 0.05
|
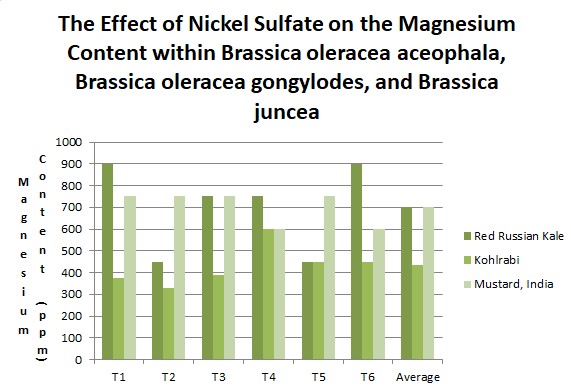
|
Table 1.2 Descriptive Information
Nickel Sulfate Group
Magnesium Content (ppm)
|
Red Russian Kale(Brassica oleracea acephala
|
Kohlrabi (Brassica oleracea gongylodes)
|
Mustard, India (Brassica juncea)
|
|
Mean
Variance
Standard Deviation
1 SD (68% Band)
2 SD (95% Band)
3 SD (99% Band)
Number
|
700
42000
204.94
495.06 – 904.94
290.12 – 1109.88
85.18 – 1314.82
6
|
432.5
8857.5
94.11
338.39 – 526.61
244.28 – 620.72
150.17 – 714.83
6
|
700
6000
77.46
622.54 – 777.46
545.08 – 854.92
467.62 – 932.38
6
|
|
Results of F-Test
|
F = 7.55
Critical F=3.6823 < 7.55
|
Df =2,15
rejected
|
p>0.10
α = 0.05
|
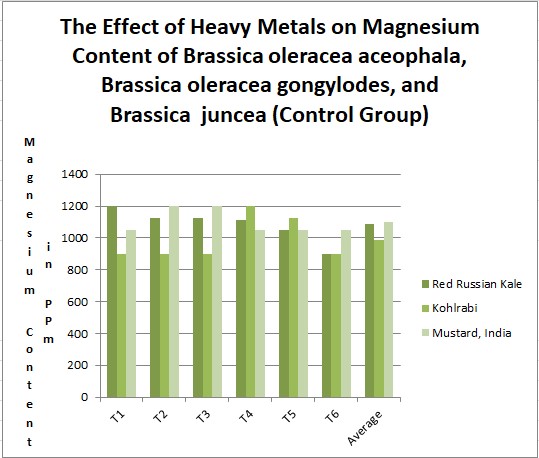
|
Table 1.1 Descriptive Information
Control Group
Magnesium Content (ppm)
|
Red Russian Kale(Brassica oleracea acephala
|
Kohlrabi (Brassica oleracea gongylodes)
|
Mustard, India (Brassica juncea)
|
|
Mean
Variance
Standard Deviation
1 SD (68% Band)
2 SD (95% Band)
3 SD (99% Band)
Number
|
1085
10500
102.47
982.53 – 1187.47
880.06 – 1289.94
777.59 – 1392.41
6
|
987.5
18937.5
137.61
849.89 – 1125.11
712.28 – 1262.72
574.64 – 1400.33
6
|
1100
6000
77.46
1022.54 – 1177.46
945.08 – 1254.92
867.62 – 1332.38
6
|
|
Results of F-Test
|
F = 3.79
Critical F=3.6823 < 3.79
|
Df =2,15
rejected
|
p>0.10
α = 0.05
|
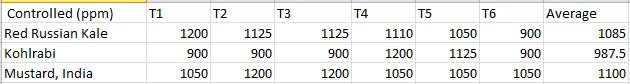
Results. Effects of heavy metal treatment on the magnesium content of the control group is summarized in Table 1.1. Brassica oleracea gongylodes (Kohlrabi) had a mean average magnesium content in parts per million of 987.5, a variance of 18937.5, and a standard deviation of 137.61. Brassica juncea (Mustard, India) had a mean average magnesium content in parts per million of 1100, a variance of 6000, and a standard deviation of 77.46. The f test was used to test the following null hypothesis at the 0.05 level of significance: There is no significant difference in the mean averages of magnesium content within Brassica juncea, Brassica oleracea aceophala and Brassica oleracea gongylodes when not treated with heavy metal. The null hypothesis was rejected (f=3.6823 < 3.79 at df=2,15; p>0.10). The data did support the research hypothesis that plants exposed to heavy metal accumulation will have a decrease in magnesium content.
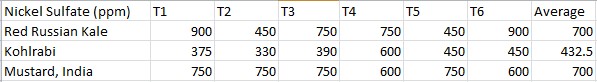
Effects of heavy metal treatment, Nickel Sulfate, on the magnesium content of the Nickel Sulfate treated group is summarized in Table 1.2. Brassica oleracea acephala (Red Russian Kale) had a mean average magnesium content in parts per million of 700 ppm, a variance of 42000, and a standard deviation of 204.94 ppm. Brassica oleracea gongylodes (Kohlrabi) had a mean average magnesium content in parts per million of 432.5, a variance of 8857.5, and a standard deviation of 94.11. Brassica juncea (Mustard, India) had a mean average magnesium content in parts per million of 700, a variance of 6000, and a standard deviation of 77.46. The f test was used to test the following null hypothesis at the 0.05 level of significance: There is no significant difference in the mean averages of magnesium content within Brassica juncea, Brassica oleracea aceophala and Brassica oleracea gongylodes when exposed to Nickel Sulfate. The null hypothesis was rejected (f=3.6823 < 7.55 at df=2,15; p>0.10). The data did support the research hypothesis that plants exposed to heavy metal accumulation will have a decrease in magnesium content.
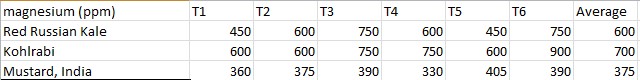
Effects of heavy metal treatment, Chromium Sulfate on the magnesium content of the Chromium Sulfate group is summarized in Table 1.3. Brassica oleracea aceophala (Red Russian Kale) had a mean average magnesium content in parts per million of 600 ppm, a variance of 1800, and a standard deviation of 42.43 ppm. Brassica oleracea gongylodes (Kohlrabi) had a mean average magnesium content in parts per million of 700, a variance of 111,000, and a standard deviation of 333.17. Brassica juncea (Mustard, India) had a mean average magnesium content in parts per million of 375, a variance of 720, and a standard deviation of 26.83. The f test was used to test the following null hypothesis at the 0.05 level of significance: There is no significant difference in the mean averages of magnesium content within Brassica juncea, Brassica oleracea aceophala and Brassica oleracea gongylodes when exposed to Chromium Sulfate. The null hypothesis was rejected (f=3.6823 < 3.84 at df=2,15; p>0.10). The data did support the research hypothesis that plants exposed to heavy metal accumulation will have a decrease in magnesium content.
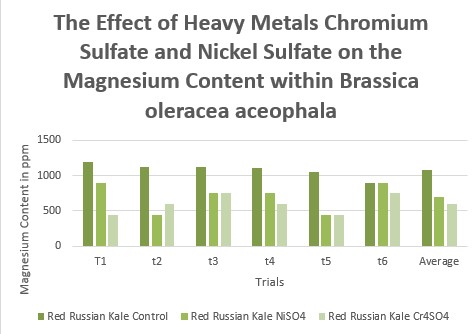
Effects of heavy metal treatment, Nickel Sulfate or Chromium Sulfate on the magnesium content of within Brassica oleracea aceophala (Red Russian Kale) is summarized in Table 1.4. Control group, untreated, Brassica oleracea aceophala (Red Russian Kale) had a mean average magnesium content in parts per million of 1085 ppm, a variance of 10500, and a standard deviation of 102.47 ppm. Brassica oleracea aceophala (Red Russian Kale), exposed to Nickel Sulfate had a mean average magnesium content in parts per million of 700, a variance of 42000, and a standard deviation of 204.94.. Brassica oleracea aceophala, treated with Chromium Sulfate, had a mean average magnesium content in parts per million of 600, a variance of 18000, and a standard deviation of 134.16. The f test was used to test the following null hypothesis at the 0.05 level of significance: There is no significant difference in the mean averages of magnesium content within Brassica oleracea aceophala when exposed to Nickel Sulfate or Chromium Sulfate. The null hypothesis was rejected (f=3.6823 < 16.74 at df=2,15; p>0.10). The data did support the research hypothesis that plants exposed to heavy metal accumulation will have a decrease in magnesium content.
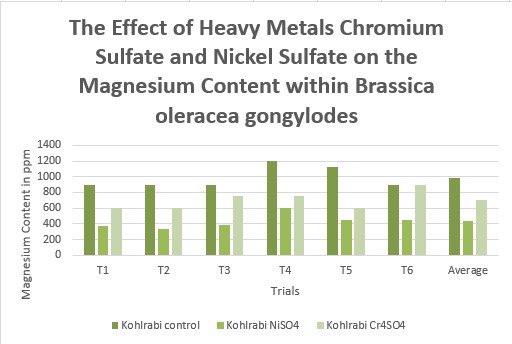
Effects of heavy metal treatment, Nickel Sulfate or Chromium Sulfate on the magnesium content of within Brassica oleracea gongylodes (Kohlrabi) is summarized in Table 1.5. Control group, untreated, Brassica oleracea gongylodes(Kohlrabi) had a mean average magnesium content in parts per million of 987.5 ppm, a variance of 18937, and a standard deviation of 137.61 ppm. Brassica oleracea gongylodes (Kohlrabi), exposed to Nickel Sulfate had a mean average magnesium content in parts per million of 432.5, a variance of 8857.5, and a standard deviation of 94.11. Brassica oleracea gongylodes (Kohlrabi) treated with Chromium Sulfate, had a mean average magnesium content in parts per million of 700, a variance of 15000, and a standard deviation of 122.47. The f test was used to test the following null hypothesis at the 0.05 level of significance: There is no significant difference in the mean averages of magnesium content within Brassica oleracea gongylodes (Kohlrabi) when exposed to Nickel Sulfate or Chromium Sulfate. The null hypothesis was rejected (f=3.6823 < 32.40 at df=2,15; p>0.10). The data did support the research hypothesis that plants exposed to heavy metal accumulation will have a decrease in magnesium content.
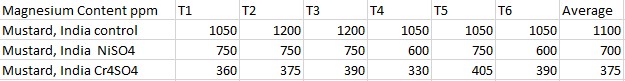
Effects of heavy metal treatment, Nickel Sulfate or Chromium Sulfate on the magnesium content of within Brassica juncea (Mustard, India) is summarized in Table 1.6. Control group, untreated, Brassica juncea (Mustard, India) had a mean average magnesium content in parts per million of 1100 ppm, a variance of 6000, and a standard deviation of 77.46 ppm. Brassica juncea (Mustard, India), exposed to Nickel Sulfate had a mean average magnesium content in parts per million of 700, a variance of 6000, and a standard deviation of 77.46. Brassica juncea (Mustard, India)treated with Chromium Sulfate, had a mean average magnesium content in parts per million of 375, a variance of 720, and a standard deviation of 26.83. The f test was used to test the following null hypothesis at the 0.05 level of significance: There is no significant difference in the mean averages of magnesium content within Brassica juncea (Mustard, India) when exposed to Nickel Sulfate or Chromium Sulfate. The null hypothesis was rejected f=3.6823 < 186.62 at df=2,15; p>0.10). The data did support the research hypothesis that plants exposed to heavy metal accumulation will have a decrease in magnesium content.
Conclusion. The effect of heavy metals, Nickel Sulfate or Chromium Sulfate on the magnesium content of Brassica oleracea acephala (RedRussianKale), Brassica oleracea gongylodes (Kohlrab) and Brassica juncea (Mustard, India) was investigated by comparing the magnesium content in parts per million after a thirteen day exposure to the heavy metals in comparison to a control group, with no exposure to heavy metals. Significant differences existed in the magnesium contents of the Brassicas exposed to Nickel Sulfate and Chromium Sulfate and the control group, no exposure to heavy metals. The research hypothesis that plants, Brassica oleracea aceophala (Red Russian Kale), Brassica oleracea gongylodes (Kohlrabi), and Brassica juncea (Mustard, India) when exposed to increased heavy metal accumulation, either Nickel Sulfate or Chromium Sulfate, will have a decrease in magnesium content. Scientists have found that inhibition of photosystem II, during photosynthesis, photoactivation, some metal ions will replace Magnesium in chlorophyll, which is a form of phytotoxicity. The possible explanations are that the Brassica genus is a hyperaccumulator, but there is a threshold for the amount of heavy metal it can uptake. Additional investigations using various other heavy metals on the Brassica genus over longer periods of time should be implemented to see if the Brassica genus is a hyperaccumulator of other heavy metals.
Applications. Fragmentation of habitats by agricultural businesses, urban development and building of infrastructure in the form of highways has led to phytotoxicity in plants. This causes environmental problems that limit plant productivity and threaten upper trophic levels. Phytoremediation can reduce this phytotoxicity caused by anthropogenic activities and provides a more environmentally beneficial and economically friendly approach to decontaminate heavy metal stressed soils. Finding plants that have evolved detoxification mechanisms is essential to ameliorate the toxic effects of heavy metals which is essential to keeping biogeochemical cycles strong and habitats resilient.
References.
(1) Ahemad, M. (2015). Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. Journal of Genetic Engineering and Biotechnology,13(1), 51-58. doi:10.1016/j.jgeb.2015.02.001
(2) Rodriguez, E., Santos, C., Azevedo, R., Moutinho-Pereira, J., Correia, C., & Dias, M. C. (2012). Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiology and Biochemistry,53, 94-100. doi:10.1016/j.plaphy.2012.01.013
(3) Shahid, M., Shamshad, S., Rafiq, M., Khalid, S., Bibi, I., Niazi, N. K., Rashid, M. I. (2017). Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere,178, 513-533. doi:10.1016/j.chemosphere.2017.03.074
(4) Vernay, P., Gauthier-Moussard, C., & Hitmi, A. (2007). Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere,68(8), 1563-1575. doi:10.1016/j.chemosphere.2007.02.052
(5) Yadav, S. (2010). Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants.
South African Journal of Botany,76(2), 167-179. doi:10.1016/j.sajb.2009.10.007















