Abstract. The objective of this study is to clarify the photophysics of deep eutectic solvents (DES). In this experiment, the two DES solvents used were choline chloride with geranic acid (CAGE) and choline chloride with valeric acid (CAVE). The results show that the original knowledge of the photophysics of deep eutectic solvents may not be correct.
Introduction. Deep eutectic solvents (DES), derived from renewable biomolecules, are heralded as sustainable alternatives to petroleum-derived solvents.1 They have many applications, such as drug delivery, chemical synthesis, and electrochemistry.2 However, their photophysics, photochemistry, and photobiology need to be clearly understood. This work sought to bridge the knowledge gap to enable photo-related applications.
Materials and Method. Different DESs were synthesized. For CAVE, we mixed one mole of choline bicarbonate with three moles of valeric acid and while fore CAGE, we mixed one mole of choline bicarbonate with three moles of geranic acid (Figure 1). The mixture was stirred at room temperature until effervescence stopped. After that, the DES was concentrated for 20 minutes under vacuum at 60 oC using rotary evaporator, then dried in a vacuum oven for three days. Then, the fluorescence of the hydrated DESs (50-200 mM) was measured using spectrofluorometer. Distilled water was used as a blank.
 Figure 1. Schematics showing the synthesis of CAGE from choline bicarbonate and geranic acid.
Figure 1. Schematics showing the synthesis of CAGE from choline bicarbonate and geranic acid.
Results and Discussion.
In this research our hypothesis was that DES undergoes unconventional photophysics. We tested the hypothesis by synthesizing two DESs, CAGE and CAVE, and evaluated their fluorescence emission. The results show that hydrated 50-400 mM CAGE feature an excitation wavelength dependent emission. The results also show emission peak upon excitation of 400 mM CAGE at 380 nm (Figure 2). This demonstrated that CAGE exhibit an unconventional photophysics since it lacks a pi-conjugated system. This result concurs with our prior report3 that show that hydrated CAGE features an unconventional photon absorption and emission. Unconventional fluorescence in small molecules lacking pi-conjugation is emerging as an alternative to the typical fluorescence of aromatic compounds.
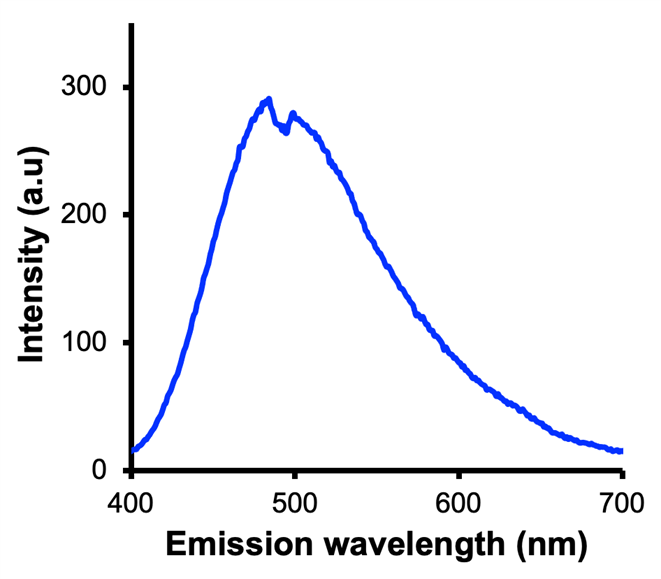
Figure 2. Emission spectrum of 400 mM of CAGE excited at 380 nm.
Conclusion. According to Stokes theory, the peaks at different wavelengths should be at the same value. However, according to these results, at different wavelengths, the peaks have different values. This goes against the theory and brings the chance for new research to be done to light.
Acknowledgments. I would like to thank Dr. Christian Agatemor for offering me the opportunity to conduct this research and Anaya Hill, Amina Denis, and Sam Oluwole for instructing me on the synthesis and using the equipment. I also thank the Young Scholar Program of the Faculty of Arts and Sciences, University of Miami, for the opportunity to conduct this research.
References.
- Agatemor, C., Quintana, A. A., Sztapka, A. M., & Santos, V. E. de C. Enabling Sustainable Chemistry with Ionic Liquids and Deep Eutectic Solvents. Angew. Chem. Int. Ed. 2022, 134, e202205609.
- Smith, E. L., Abbott, A. P., Ryder, K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060.
- Oluwole, S. A., Veríssimo, N. V., Denis, A. A., Garcia, N. T., Fura, S., Jayaraman, K., Valles, J. D., Hernandez Del Rosario, D. Patel, P. N., Duran, A., Hakim, Q. A., Aline Quintana, A., Agatemor, C. Unusual Photophysics of Geranic Acid Deep Eutectic Solvents. Chem. Commun. 2023, 59, 10492.





